
FY 2024 - FDA Medical Device Registration Fees
FDA Medical Device Establishment registration fee for the year 2024 is USD 7,653. FDA fiscal year 2024 starts on October 1, 2023, and ends on September 30, 2024. The annual establishment registration fee must be paid between October 1, 2023, and December 31, 2023. The establishment registration fee is not eligible for a reduced small business fee.
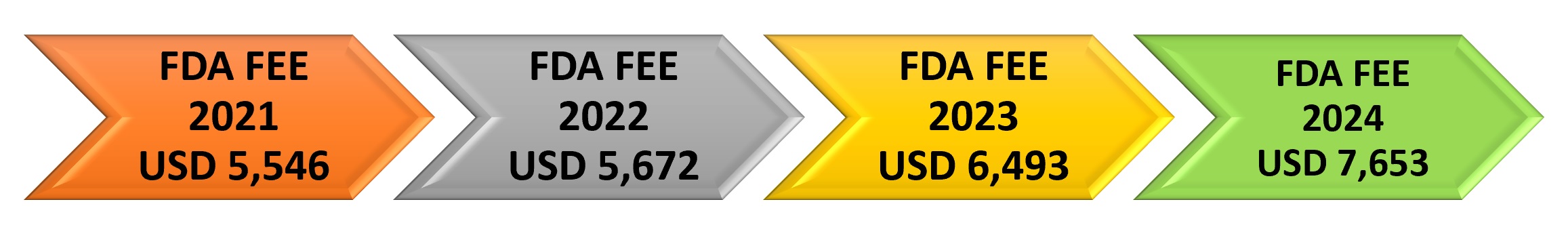
FDA Fee for Medical Device Registration and Applications for Fiscal Year 2024
Type of Registration / Application |
Standard Fees |
Small Business Fee |
Medical Device Establishment Registration |
$7,653 |
$7,653 |
FDA 510(k) Submission |
$21,760 |
$5,440 |
FDA 513(g) Submission |
$6,528 |
$3,264 |
FDA PMA, PDP, PMR, BLA Submission |
$483,560 |
$120,890 |
De Novo Classification Request |
$145,068 |
$36,267 |
Panel-track Supplement |
$386,848 |
$96,712 |
180-Day Supplement |
$72,534 |
$18,134 |
Real-Time Supplement |
$33,849 |
$8,462 |
BLA Efficacy Supplement |
$483,560 |
$120,890 |
30-Day Notice |
$7,737 |
$3,869 |
Premarket report |
$483,560 |
$120,890 |
Annual Fee for Periodic Reporting on a Class III device (PMAs, PDPs, and PMRs) |
$16,925 |
$4,231 |
FDA defines a small business as a business with $100 million or less in gross receipts or sales, including receipts or sales from its affiliates. In addition, if a small business has gross receipts or sales of $30 million or less, it is eligible to have the fee waived for its first PMA, PDP, PMR, or BLA.
Quick links
- Premarket Notification(510k)
- Establishment Registration
- Medical Device Labeling
- ISO 13485 certification
- CE Marking
- 21 CFR 820
LIBERTY MANAGEMENT GROUP LTD.
75 Executive Drive, Suite 114
Aurora, Illinois, USA - 60504
Phone : +1 (630) 270-2921
Fax : +1 (815) 986-2632
E-mail : info@fdahelp.us
